AP Chemistry 🧪
269 resourcesSee Units
Multiple Choice Practice for Acids and Bases
Welcome to Unit 8 AP Chemistry Multiple Choice Questions! Grab some paper and a pencil 📄 to record your answers as you go. You can see how you did on the Unit 8 Practice Questions Answers and Review sheet once you're done. Don't worry, we have tons of resources available if you get stumped 😕 on a question. And if solo study is not your thing, join a group in Hours!
Not ready to take a quiz yet? Take a look at the Intro to Unit 8.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and, although they cover information outlined in the AP Chemistry Course and Exam Description, the formatting on the exam may be different.
1. Which of the following correctly identifies why nitric acid is a stronger acid than nitrous acid?
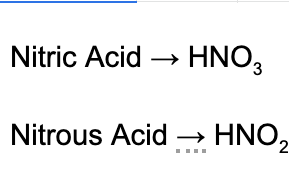
A. Nitric acid contains more oxygen atoms, which strengthen the bond to hydrogen.
B. Nitric acid contains more oxygen atoms which weaken the bond to hydrogen.
C. The Ka for nitrous acid is larger than the Ka for nitric acid.
D. Nitrate is a stronger conjugate base than nitrite.
2. What is the conjugate base of hydrogen phosphate?
A. Phosphate (PO4)
B. Dihydrogen Phosphate (H2PO 4-)
C. Phosphoric Acid (H2PO4)
D. Hydrogen Phosphate (HPO3 2-)
3. Which of the following statements regarding methylamine is correct?
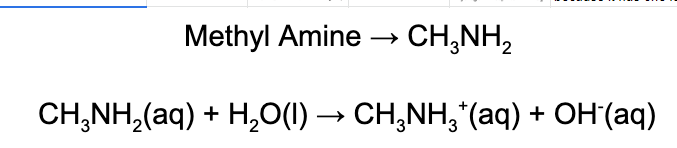
A. Methyl amine is a Bronsted-Lowry base because it accepts a proton from water.
B. Methyl amine is a Bronsted-Lowry base because it gives a proton to water.
C. Methyl amine is a Arrhenius acid because it creates hydroxide in an aqueous solution.
D. Methyl amine is a Lewis base because it makes hydroxide in an aqueous solution.
4. In an aqueous solution, the hydrogen ion concentration is 2.4x10^-3 M. What is the pOH?
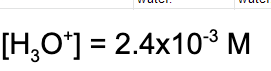
A. 2.62
B. 11.38
C. 2.40
D. 11.60
5. What is the pH of a 0.00034 M solution of sodium hydroxide (NaOH)?
A. 3.47
B. 10.53
C. 2.47
D. 11.53
6. What is the pH of a 0.55 M solution of acetic acid?
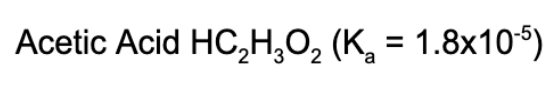
A. 9.00
B. 5.00
C. 11.50
D. 2.50
7. What is the net ionic equation for the reaction between hydrofluoric acid and potassium hydroxide?
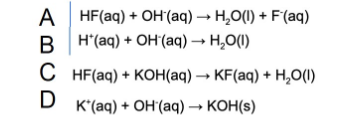
8. Which of the following has the highest pH?
A. Solution containing equimolar amounts of NaOH and HCl
B. 1.0 M solution of HCl
C. 1.0 M solution of NaOH
D. 1.0 M solution of HF (Ka = 0.00018)
9. Which of the following combinations of substances is a buffer solution?

10. 25.0 mL of 1.0 M acetic acid is mixed with 25.0 mL of 1.0 M sodium hydroxide. What is the resulting pH?
A. pH = 7
B. pH > 7
C. pH < 7
D. pH = 0
11. A solution is prepared such that the concentration of formic acid is 0.024 M and the concentration of sodium formate is 0.103 M. What is the pH of the reaction mixture?
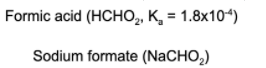
A. 7.00
B. 9.62
C. 1.62
D. 4.38
12. Which of the following salts is the most acidic?

13. Which of the following would be the best indicator to observe a neutralization reaction between KOH and HBr?
A. Methyl Orange (pKa = 4.95
B. Phenolphthalein (pKa = 9.4)
C. Thymol Blue (pKa = 1.65)
D. p-Nitrophenol (pKa = 7.2)
14. What is the concentration of 1.55 M NaOH is required to titrate 24 mL of a 0.75 M HI solution?
A. 11.6 mL
B. 49.6 mL
C. 11.6 L
D. 49.6 L
15. When a small amount of HCl is added to a solution containing equimolar amounts of NaF and HF, which of the following statements is true?
A. [NaF] < [HF] because the HCl reacts with the conjugate base.
B. [NaF] > [HF] because the HCl reacts with the conjugate base.
C. [NaF] < [HF] because the HCl reacts with the conjugate acid.
D. [NaF] > [HF] because the HCl reacts with the conjugate acid.
- 🙌 Time to check your answers on Unit 8 Practice Questions Answers and Review
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.