AP Chemistry 🧪
269 resourcesSee Units
Answers and Review for Multiple Choice Practice on Molecular and Ionic Compound Structures and Properties
⛔STOP ! ⛔ Before you look at the answers, make sure you gave this practice quiz a try so you can assess your understanding of the concepts covered in Unit 4. Click here for the practice questions: AP Chemistry Unit 4 Multiple Choice Questions

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and although they cover information outlined in the AP Chemistry Course and Exam Description the formatting on the exam may be different.
1. Which of the following best describes what happens when water boils at temperatures above 100°C?
A. A chemical change occurs because chemical bonds are being broken and formed.
B. A chemical change occurs, because no chemical bonds are being broken.
C. A physical change occurs, because no chemical bonds are being broken.
D. A physical change occurs because chemical bonds are being broken and formed.
Answer: Water boiling is an example of a physical process because no new substance was formed. A chemical reaction is defined as bonds being formed or broken and a new substance being created.
📄 Study AP Chemistry, Unit 4.4: Physical vs. Chemical Changes
2. What is the net ionic equation for the following chemical reaction?
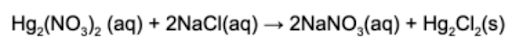
A.
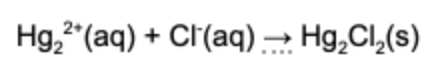
B.
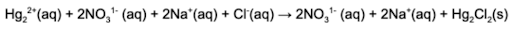
C.
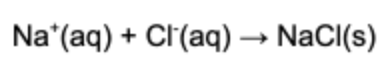
D.
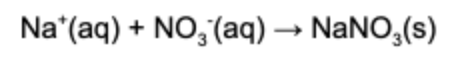
Answer: The net ionic reaction refers to the ionic species that react and removes all the spectator ions. The spectator ions are the species in the reaction that remain dissolved in solution.
📄 Study AP Chemistry, Unit 4.8: Intro to Acid-Base Neutralization Reactions
3. When 1.50 mole of hydrogen reacts with 0.50 mole of oxygen, how many grams of water are produced?
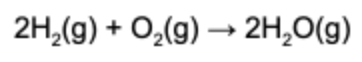
A. 1 g water
B. 1.5 g water
C. 18 g water
D. 27 g water
Answer: The limiting reactant is oxygen, since it will be consumed by the reaction completely first. When 0.5 mole of oxygen reacts with an excess of hydrogen, 1.0 mole of water is produced. Lastly, water has a molar mass of 18 g/mol, so the mass of water is 18 g.
📄 Study AP Chemistry, Unit 4.8: Intro to Acid-Base Neutralization Reactions
4. What type of reaction is represented by the balanced chemical equation below?
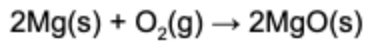
A. Acid-Base Reaction
B. Oxidation-Reduction Reaction
C. Precipitation Reaction
D. Decomposition Reaction
Answer: This is a oxidation-reduction (redox) reaction, because electrons were transferred from magnesium to oxygen. One way redox reactions can be identified, is when neutral elements make ionic compounds.
📄 Study AP Chemistry, Unit 4.9: Redox Reactions
5. Which piece of laboratory equipment is used to determine the amount of base required to completely neutralize an acid?
A. Photospectrometer
B. Filter and Filter Paper
C. Mass Spectrometer
D. Burette
Answer: A burette is a piece of glassware that precisely measures the amount of solution is added to a reaction vessel or flask. Acid-base reactions require a burette to know the precise amount of titrant (base) that was added to neutralize the analyte (acid).
📄 Study AP Chemistry, Unit 4.6: Titrations - Intro and Calculations
6. What is the oxidation number of nitrogen in nitric acid?

A. -3
B. 0
C. 3
D. 5
Answer: Oxygen has an oxidation number of -2 in most molecules and hydrogen has an oxidation number of +1. The oxidation states of the elements must add to the overall charge of the molecule; therefore, nitrogen must have an oxidation state of +5.
📄 Study AP Chemistry, Unit 4.9: Redox Reactions
7. What is the conjugate acid of hydrogen phosphate?

A.
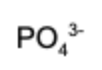
B.

C.
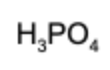
D.
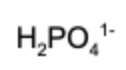
Answer: A conjugate acid has exactly one more hydrogen than the base it's compared to. Therefore, dihydrogen phosphate is the conjugate acid.
📄 Study AP Chemistry, Unit 4.8: Intro to Acid-Base Neutralization Reactions
8. Which of the elements is oxidized in the following unbalanced, redox reaction?
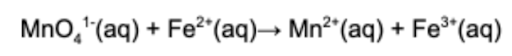
A. Iron (Fe)
B. Manganese (Mn)
C. Oxygen (O)
D. Hydrogen (H)
Answer: Oxidation occurs when a substance loses electrons. Iron loses electrons and this is evident based on the fact that iron becomes more positive.
📄 Study AP Chemistry, Unit 4.9: Redox Reactions
9. When 44.8 L of hydrogen gas is produced at STP, what mass of zinc reacted?
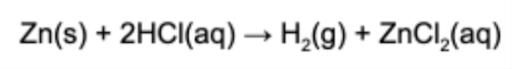
A. 2.00 g
B. 65.4 g
C. 131 g
D. 1460 g
Answer: At STP, one mole of a gas occupies 22.4 L. Therefore, 2.00 mol of hydrogen gas was produced. Zinc produces hydrogen in a 1 mol : 1 mol ratio. Lastly, the molar mass of zinc is 65.4 g/mol.
📄 Study AP Chemistry, Unit 4.5: Stoichiometry & Calculations
10. When 2.5 mol of methane reacts, 45 g of water is produced. What is the percent yield of the reaction?
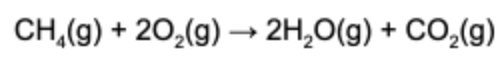
A. 5.60%
B. 25%
C. 50%
D. 100%
Answer: 2.5 moles of methane reacts to produce 5.0 moles of water. By converting this to mass, we find that the theoretical yield is 90 g. Since only 45 g of water was collected. the percent yield is 50%.
📄 Study AP Chemistry, Unit 4.0: Unit 4 Overview: Chemical Reactions
11. Which of the following describes the types of reaction with an appropriate justification?
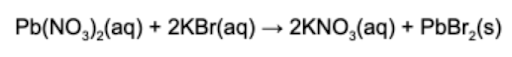
A. This is an acid base reaction because a proton is transferred between the reactants.
B. This is a precipitation reaction because a solid is produced.
C. This is an redox reaction because an electron is transferred between the reactants
D. This is a combustion reaction because oxygen is present.
Answer: Precipitation reactions are when a solid is produced when solutions mix. Lead (II) bromide is the precipitate of the reaction described.
📄 Study AP Chemistry, Unit 4.7: Precipitation Reactions
12. What is(are) the product(s) of the reaction if solid magnesium and aqueous copper chloride are mixed?
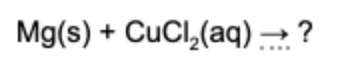
A.
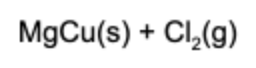
B.
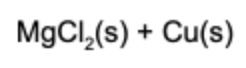
C.
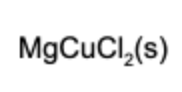
D. No reaction occurs
Answer: This is a single replacement reaction. Magnesium combines with chlorine and copper solidifies in the solution.
📄 Study AP Chemistry, Unit 4.7: Precipitation Reactions
13. What amount of 1.0 M HCl must be added to a 40. mL of 2.0 M LiOH in order to completely neutralize the solution?
A. 15 mL
B. 20 mL
C. 40 mL
D. 80 mL
Answer: Since HCl and LiOH react in a 1 mol : 1 mol ratio, one can use the titration equation in order to determine the amount of HCl that reacts with LiOH.
📄 Study AP Chemistry, Unit 4.6: Titrations - Intro and Calculations
14. What element is reduced in the following unbalanced, chemical reaction?
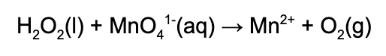
A. Manganese (Mn)
B. Oxygen (O)
C. Hydrogen (H)
D. Reaction needs to be balanced
Answer: A reduction occurs when a substance gains electrons. This is noted when the element gets more negative. Manganese gained electrons and therefore is reduced.
📄 Study AP Chemistry, Unit 4.3: Balancing Chemical Equations
15. Which piece of laboratory equipment is used to collect a precipitate?
A. Crucible
B. Filter and Filter Paper
C. Mass Spectrometer
D. Bunsen Burner
Answer: In order to collect a precipitate, the solid must be collected via filter paper. All other methods listed are not appropriate for collecting a solid.
📄 Study AP Chemistry, Unit 4.8: Intro to Acid-Base Neutralization Reactions
What can we help you do now?
- 🔍Check out all of the resources for AP Chem Unit 5
- 🦘Jump to AP Chem Unit 5 Multiple Choice Questions
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.