Unit 1 FRQ (Photoelectron Spectroscopy) with Feedback
2 min read•july 11, 2024
Wes Winter
Wes Winter
AP Chemistry 🧪
269 resourcesSee Units
AP Chemistry Free Response Question for Electron Spectroscopy
Consider the following photoelectron spectroscopy data:
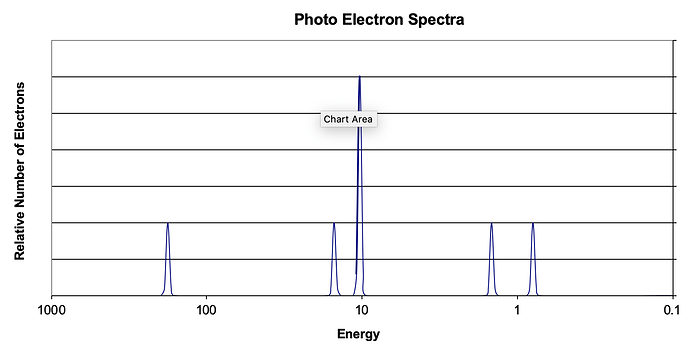
a) Based on the graph, identify the element and write the full electron configuration.
b) A student claims that the 1s peak for sulfur (S) would be further to the right than the 1s peak of this element. Is this student correct? Justify your answer.
FRQ Writing Samples & Feedback
FRQ Practice Submission 1
a) 1s^2 2s^2 2p^6 3s^2 3p^2 Silicon
b)This student is incorrect because the distance between the 1s orbital and the nucleus for a sulfur atom is less than the distance for a silicon atom due to the increased number of protons in sulfur that allow for a strong attraction and hold on the inner electrons. This means that more energy would be required for an s orbital electron to be removed and the 1s peak would be further left than the 1s peak for Silicon.
Teacher FRQ Feedback
I think the only change would be referencing the attractive force to the electrons to the nucleus, not the distance (Though true). A better statement would sound "Since sulfur has more protons than silicon, Sulfur has a greater nuclear charge, thus a greater attraction to the 1s electrons. Nice!
FRQ Practice Submission 2
a) Silicon is the element. The electron configuration is: [Ne] 3s^2 3p^2.
b) The student is incorrect - the 1’st peak for sulfur would NOT be further to the right than the 1st peak for silicon. This is because sulfur has a smaller atomic radius and a higher electronegativity than silicon. All of these factors would result in a higher amount of energy required for sulfur in the photo electra spectra because sulfur has greater attractions to the nucleus compared to silicon, making it harder for electrons to be removed.
Teacher FRQ Feedback
Your statement in regards to 1b) is correct, however your justification is off. What can be frustrating about this section is when to use which justification. The biggest reason that the 1s peak for sulfur would be further to the left is because the nuclear charge (number of protons) for sulfur is greater. While your other statements are true, they do not correctly justify the location of the 1s peak. Hope this helps.
Teacher's Solutions
a) The element is silicon. It has an electron configuration of 1s22s22p63s23p2
b) This student is incorrect. The 1s peak for sulfur would be further to the left on the photoelectron spectra. This is due to the fact that sulfur has more protons in the nucleus and therefore has a greater nuclear charge. This greater positive charge attracts the electrons in sulfur greater, thus moving the peak further to the left.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

© 2024 Fiveable Inc. All rights reserved.