What is the trend for atomic radius?
1 min read•january 11, 2021
AP Chemistry 🧪
269 resourcesSee Units
Periodic Trends: The Atomic Radius
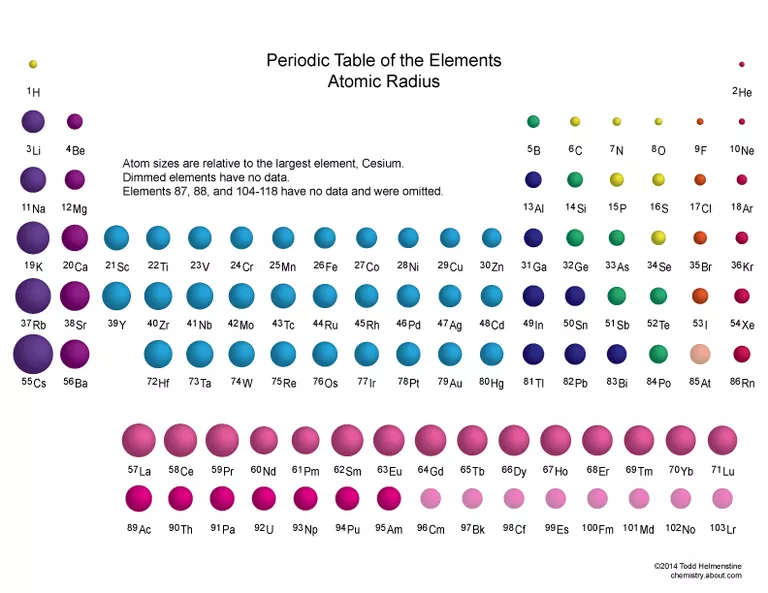
There are two things you should know about the periodic trend for atomic radius:
First, atomic radius increases as you go down the periodic table.
- The higher the period, the higher the energy level. Energy levels are represented by the coefficients when you write electron configurations (ex. the 1 in 1s², the 2 in 2p⁶, etc.).
- Electrons in the highest energy level are farthest away from the nucleus, so the size of the electron cloud increases going down the periods.
Second, atomic radius decreases from left to right across a period.
- This is because the effective nuclear charge increases with the addition of more protons.
- Effective nuclear charge is the net positive charge felt by valence electrons, estimated by (number of protons) - (inner electrons). In carbon, for example, the effective nuclear charge is +4 (6 protons minus 2 electrons in the first shell).
- In short, because you're adding protons, electrons feel a stronger attraction to the nucleus and the size of the atom decreases.
- Watch this 🎥 video on all of the periodic table trends
- For a quick review read this study guide on Unit 1: Atomic Structure and Properties
Check out these reviews for more help on periodic trends and the table of elements:
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.